Authors: D’Ambra N. Dent, BA; Stacey A. Ingram, MEd; Valerie M. Lawhon, BS; Julie Scott RN, MSN; Nadia Still DNP, RN; Debra Wujcik, PhD, RN, FAAN; Carevive PROmpt®, Gabrielle B. Rocque, MD, MSPH; Smith Giri, MD,MHS Omer Jamy, MD
University of Alabama at Birmingham; Carevive Inc.
Background
- Home-based symptom monitoring using patient-reported outcomes has been shown to:
- Reduce symptom burden and hospitalizations
- Improve quality of life and overall survival
Objective
The primary goal of this study was to evaluate the early feasibility of home-based symptom monitoring.
Methods
Home-based symptom monitoring
- Participants enrolled into Carevive PROmpt® symptom monitoring with the option to use of text, email, or both to complete weekly symptom surveys.
- Patients receive an auto-generated self-management plans based on their electronic patient-reported outcomes (ePRO) responses.
- Nurses respond to symptom alerts
Study Design
- Prospective single-arm pilot study evaluating feasibility of a two-part education and technology intervention (home-based symptom monitoring) for clinicians treating patients with Multiple Myeloma (MM) and Acute Myeloid Leukemia (AML) patients
Participants
- Patients aged 60 and older with MM or AML
- Newly diagnosed or starting a new line of therapy and have not yet made a treatment decision
Data Collection
- Patients received surveys between September 1, 2020 –May 19, 2021.
- Chart abstraction in Carevive PROmpt® platform
Analysis
- Descriptive statistics were calculated for the following outcomes:
- Compliance rate
- Completion rate
- Survey alert type and frequency
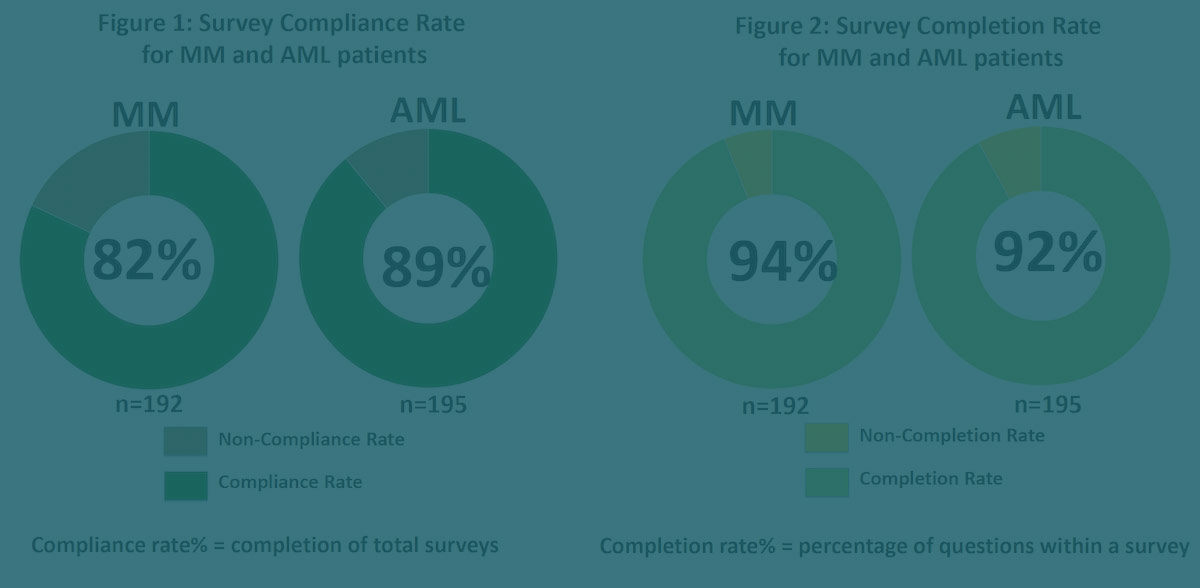
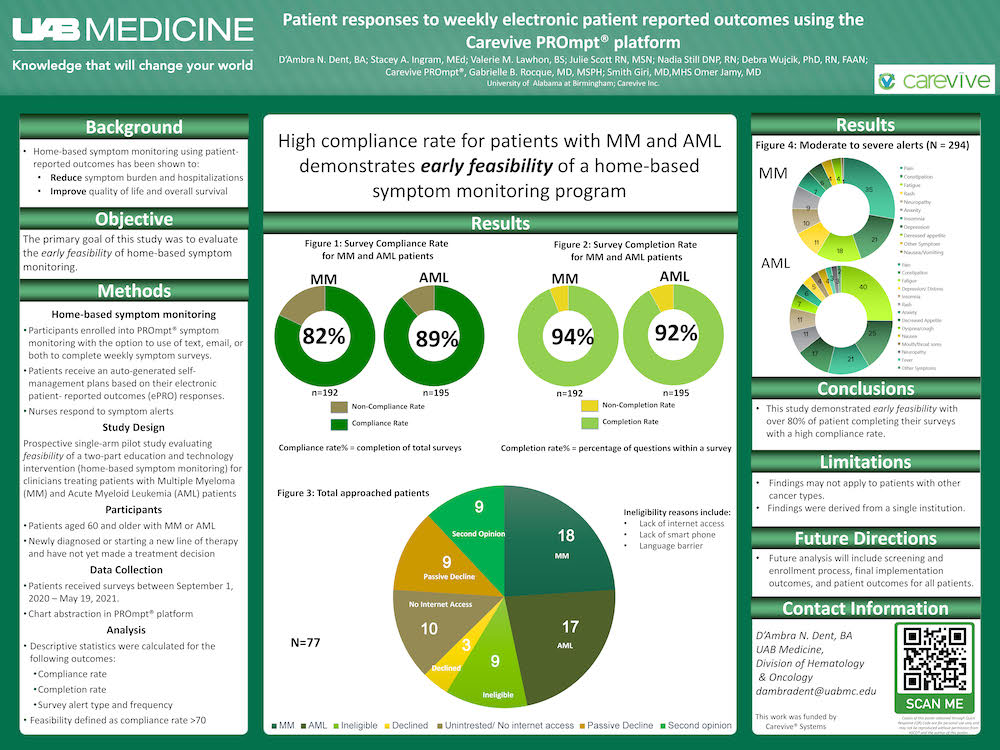
- Feasibility defined as compliance rate >70
Conclusion
This study demonstrated early feasibility with over 80% of patient completing their surveys with a high compliance rate.
Limitations
- Findings may not apply to patients with other cancer types.
- Findings were derived from a single institutions.
Future Directions
- Future analysis will include screening and enrollment process, final implementation outcomes, and patient outcomes for all patients.