Authors: Nia M. Mitchell1, Susan C. Locke2, Kris W. Herring2, Aaron Galaznik3, Debra Wujcik3, Thomas W. LeBlanc2,4
1Duke University School of Medicine, 2Duke Cancer Institute, 3Carevive Systems Inc., 4Division of Hematologic Malignancies and Cellular Therapy, Dept. of Medicine, Duke University School of Medicine
Background
- Lack of real-world data on patient experience of acute myeloid leukemia (AML) treatment since advent of novel therapies
- Increased utility of routine symptom monitoring using patient-reported outcome measures (PROMs)
- Prospective observational study using Patient Reported Outcomes mobile platform (PROmpt®)—facilitates reporting of symptoms and level of severity (none, mild, moderate, severe, or very severe) to healthcare team via weekly surveys while on treatment
Methods
- Enrolled patients with AML treated at Duke to use the PROmpt® system between 1/1/2022–4/19/2023
- Analyzed reported symptoms over 12-week period using descriptive statistics, with a focus on moderate to very severe (Mod-VS) symptoms in patients undergoing low-intensity vs. high-intensity treatment regimens
Results
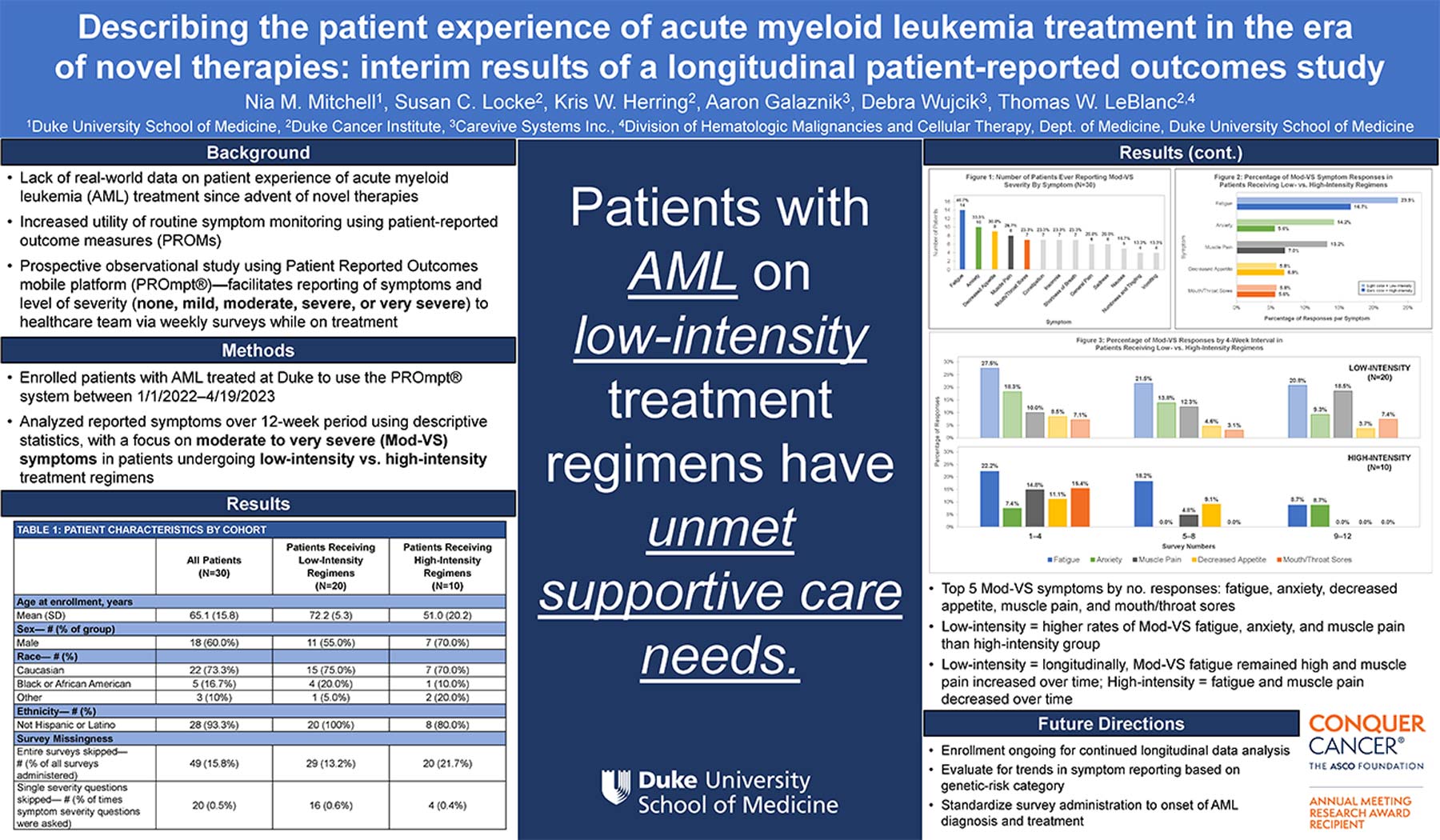
- Top 5 Mod-VS symptoms by no. responses: fatigue, anxiety, decreased appetite, muscle pain, and mouth/throat sores
- Low-intensity = higher rates of Mod-VS fatigue, anxiety, and muscle pain than high-intensity group
- Low-intensity = longitudinally, Mod-VS fatigue remained high and muscle pain increased over time; High-intensity = fatigue and muscle pain decreased over time
Future Directions
- Enrollment ongoing for continued longitudinal data analysis
- Evaluate for trends in symptom reporting based on genetic-risk category
- Standardize survey administration to onset of AML diagnosis and treatment